ORIGINAL
Supplementation with humic substances affects the innate immunity in layer hens in posfasting phase
El suplemento de sustancias húmicas afecta la inmunidad innata en gallinas ponedoras en fase posmuda
Rosa Sanmiguel P,1* M.Sc, Iang Rondón B,2 M.Sc.
1Universidad Cooperativa de Colombia, Faculty of Veterinary and Zootechnical Sciences, Laboratory of Morphophysiology, IMPRONTA Research Group. Calle 14 número 107-59, barrio El Salado, Ibagué, Colombia.
2Universidad del Tolima, Faculty of Veterinary and Zootechnical Sciences, Veterinary Diagnostics Laboratory, Inmuno-biology and Pathogenesis Research Group - GIP. Bloque 33 L105, barrio Santa Helena, Ibagué, Colombia.
*Correspondence: rosa.sanmiguel@campusucc.edu.co
Received: July 2014; Accepted: February 2015.
ABSTRACT
Objective. Asses the effect of supplementation with Humic substances (HS) over some innate immunity parameters (serum bactericidal activity, phagocytosis, bacterial agglutination, respiratory burst and lisozyme activity) in phase after fasting of layer hens. Materials and methods. 120 posfasting phase Hy Line Brown layer hens were taken which were distributed into four groups: The first and the second were supplemented with 0.1 and 0.2% of HS, respectively. The third group was supplemented with 0.25 mg/kg on levamisole hydrochloride and fourth group have no supplementation; during sixty days period. Blood samples were collected on 8th, 30th and 60th of experiment day. Results. The phagocytic index and respiratory burst increased significantly at day 30th in HS supplemented groups. Alike, serum bactericidal activity and lisozyme activity improved on 8 th day, nevertheless, changes were no evident latter. The bacterial agglutination was high in supplemented groups evaluated at everyone times. Conclusions. Results showed that HS behave as immunostimulant in the early phase after fasting layer hens.
Key words: Agglutination, bactericide activity, Lisozyme, phagocytosis (Source: DeCS).
RESUMEN
Objetivos. Evaluar el efecto de las sustancias húmicas (SH) sobre algunos parámetros de la inmunidad innata (actividad bactericida del suero, fagocitosis, aglutinación bacteriana, explosión respiratoria y actividad de la lisozima) en la fase posmuda de gallinas ponedoras. Materiales y métodos. Se utilizaron 120 gallinas ponedoras Hy Line Brown en la fase de posmuda, las cuales fueron divididas en cuatro grupos: Los dos primeros fueron suplementados con 0.1 y 0.2% de SH respectivamente, el tercer grupo fue suplementado con 0.25 mg/kg de Clorhidrato de levamisol y el cuarto grupo control sin suplemento; durante un período de 60 días. Las muestras sanguíneas se tomaron los días 8, 30 y 60 del experimento. Resultados. El Índice fagocítico y la explosión respiratoria se incrementaron significativamente a partir del día 30 de suplementación con SH. De la misma manera, la actividad bactericida del suero y la actividad de la lisozima aumentaron al día 8; no obstante no se evidenciaron cambios posteriores. La aglutinación bacteriana fue significativamente mayor en los grupos suplementados en todos los tiempos evaluados. Conclusiones. Los resultados demuestran que las SH se comportan como agentes inmunoestimulantes en la fase temprana de la posmuda en gallinas ponedoras Hy Line Brown.
Palabras clave: Actividad bactericida, aglutinación, fagocitosis, lisozima (Fuente: DeCS).
INTRODUCTION
During an infection the immune response, whether innate or adaptive, requires an expenditure of energy that can interfere with the production capacity of susceptible animals. Traditionally, handling infectious problems also includes reducing risk factors associated with infection, and pharmacological treatment has mainly been through the use of antibiotics, with the subsequent development of resistance that has been reported (1) coupled with a limited number of new molecules. Additionally, it has been shown that the use of antibiotics can have deleterious effects on the immune response (2). For this reason, we have opted for strategies that increase the immune response of animals against infectious challenges in the environment rather than directly attacking the agent. Thus, there has been a growing use of immunostimulant or immunomodulatory substances that have had a positive impact on the immunological parameters, minimizing the negative effects from an economic, productive and environmental standpoint (3).
In birds, several studies report the use of immunostimulatory substances, including levamisole, probiotics, prebiotics, bursa of Fabricius and thymus extract, vaccine products (3-5).
Humic substances (SH) are the end product of the biotransformation of organic matter, among which the most common are humic and fulvic acids and melanin (6). Although these substances are not reported in the tables of additives in animal diets, some studies show that they have an important potential to promote growth in broilers and laying hens (7). This could improve productive performance, carcass traits and egg quality. This can even be observed in the final stage of the production cycle, when physiologically a decrease in production and product quality occurs, effects that are diminished when the diet is supplemented with SH (8,9).
In the poultry industry there are different methods to extend the productive life of laying hens; a common one is inducing molt (10). The most common methods is the conventional molt program through prolonged fasting with weight loss that leads to a goal weight obtained at 18 weeks. After a complete cessation of egg production for at least 2 weeks, an improved production is achieved, since the laying is improved, as well as egg and shell quality (11). However, these conditions significantly reduce fertility and hatchability in breeding hens (12).
It has been reported that the stressful nature of molting adversely affects immune response and, in contrast, that the immune response adversely affects molting (13). In this regard, to the extent that stress generated by molting increases adrenal corticosteroids plasma levels, the immune system decreases its effectiveness by reducing the total leukocyte count and the different cellular and antibody responses (13,14).
It is also noteworthy that growth performance depends on factors such as nutritional status, health status and immune performance. However, despite the existence of reports on the effects of SH on some production parameters, its effect on the immune response in different phases of production is unknown. This study evaluated the effect of dietary supplementation with 0.1 and 0.2% humic acids on some parameters of innate immunity (serum bactericidal activity, phagocytic index, bacterial agglutination, respiratory burst, lysozyme activity) in commercial laying hens in the post-molt phase.
MATERIALS AND METHODS
Experimental conditions. This study was conducted in installations for laying hens on the experimental farm of the Universidad Cooperativa de Colombia in El Salado, Ibague, Colombia, 03°24”N74°56”. The average temperature in the study area was 28.2°C.
Ethical aspects. The experiment was carried out under regulations for animal production and welfare endorsed by the Committee on Bioethics of the Universidad de Tolima under the central investigation committee. To conduct this study, the corresponding authorization from the Ethics Committee for Research of the Universidad de Tolima was requested, and a minimum statistical sample of birds (120) was used with 95% confidence, and breeding and sample taking was performed by expert staff to minimize the stress caused by the experiment.
Experimental animals. According to the formula of finite populations, 120 clinically healthy Hy Line Brown laying hens in post-molt phase were used, which had been previously vaccinated against Gumboro (Lukert strain), Infectious Viral Bronchitis (Massachusetts strain) and New Castle (B1 strain) from the Laverlam laboratory. The hens were kept on a raised floor, were moved to cage at 16 weeks and underwent traditional molt with prolonged fasting for 12 days and received 5g of calcium carbonate supplements per bird during fasting.
Humic substances. Humic substances subjected to the experiment were obtained from the biotransformation of cachaza, a product of sugarcane and alcohol. This underwent a biotechnological process to transform and stabilize organic matter (80% of humic acids) to obtain a pH of 6.4, density of 0.73g/cm3, maximum humidity of 12.32%, and 62.45% ash. This product was supplied by PBA Biotechnology Products.
Experimental design. A completely randomized design was used where 120 chickens were divided into four groups for eight weeks in the post-molting phase. Two treatments were supplemented with humic substances (0.1 to 0.2%), and a positive control group was supplemented with levamisole (levamisole hydrochloride 4%) administered in drinking water at a concentration of 0.25 mg/kg day, as well as a negative control group without diet supplements. In each treatment there were 10 experimental units (EU), each comprising 3 hens placed in individual cages of 20 cm x 40 cm x 30 cm in a three level pyramidal module. In all cases the water was supplied automatically ad libitum.
Day zero for the experiment was the first post-molt day, once production dropped to 0% feed consumption was started. Blood samples for the corresponding hematologic and innate immunity tests were taken on day 8, 30 and 60 of the experiment.
Sample collection. Hens were immobilized by trained staff to obtain blood samples by puncturing the metatarsal vein after disinfecting the area with 70% ethyl alcohol. Disposable syringes, 1 ml 27G x ½, were used. The collection was made in microtubes 1.5 ml with EDTA and dry microtubes (0.3 and 0.7 ml of blood respectively). The phagocytic index and respiratory burst response of white blood cells was evaluated in the blood collected in microtubes with EDTA. The blood collected in microtubes was dry centrifuged at 5000 g for 5 minutes, the serum was grouped according to each experimental unit to evaluate bactericidal activity, lysozyme, and bacterial agglutination.
Phagocytic activity test. Whole blood was kept at room temperature and diluted 1:20 in a Hanks Balanced Salt Solution (reference 14175079; Gibco/Invitrogen) with 1% NaCl. Then 60 ul of the dilution was placed in a chamber slide (Lab-TekII Chamber Slide, 8 wells) along with 250 ul of a E. coli suspension of avian origin to an optical density of 0.80 (1 x 108 CFU). The micro preparation was done in duplicate and incubated for 15 minutes at 40.5°C. The phagocytic process was stopped by cooling on iced sheets as described by Millet et al (15). It was washed with Hanks saline solution in each chamber and then they were fixed with 300 µl of methanol (99.9% pure) on ice for 5 min, stained with Hemacolor and at least 100 cells per chamber were counted as suggested by Millet et al (15). The results were interpreted as a phagocytic index (IF): Percentage of heterophiles containing bacteria X the average number of bacteria that have been ingested by heterophiles (16).
Respiratory burst test. It was performed following the spectrophotometric method described by Mohanty & Sahoo (17). Briefly, 50 µl of blood was placed in a vial with 50 µl of nitro blue tetrazolium (NBT tablet) 0.2% and incubated for 30 minutes at 25°C. Then 1 ml of NN-dimethylformamide was added at 99.8% (reference 161785) to solubilize the reduced formazan and this mixture was centrifuged at 2000 x g for 5 minutes. Then 200 µl of the supernatant was taken in duplicate and plated in a U bottom multiwell plate to read the optical density in an ELISA plate reader at 650 nm. 200 µl of NN-dimethyl formamide was used as a target.
Lysozyme activity test. For this test, a bacterial suspension of Micrococcus luteus (lisodeikticus) ATCC 4698 (Reference M3770; Sigma Aldrich, Germany) was prepared to an optical density of 0.60 (1 X 108 CFU). In a multiwell plate with 96 wells 20 µl of serum from the pool of each experimental unit was placed and 180 µl of suspension was added. Reading was performed using a spectrophotometer with a 650 nm filter and was repeated after 10 minutes of incubation at room temperature (15).
Bacterial agglutination test. A 1:2 serial dilution was performed with 25 µl of serum to equal volumes of PBS and 25 µl of E. coli solution (108 cells/ml) that was previously inactivated with formalin in a multiwell U bottom plate. The plates were incubated during the night at room temperature and titers were calculated as the reciprocal of the serum dilution that had the highest complete agglutination of the bacterial cells (18).
Serum bactericidal activity test. This test was performed using the technique described by Low and Sin (19). Briefly, blood was collected without anticoagulant and kept at room temperature for one hour. Subsequently, it was centrifuged at 2500 g for 5 minutes and the supernatant serum was removed. A bacterial suspension in PBS was prepared from E. coli of avian origin to an optical density of 0.80, which was diluted 1:10 3 times serially.
2 µl of the diluted suspension of the bacteria was incubated with 20 µl of the serum in a vial for 1 hour at 37°C. PBS was used for the control group to replace blood serum. It was massively planted in Trypticase Soy Agar (TSA) and incubated at 37°C for 24 hours, after which UFC counting was done.
Statistical analysis. The response variables were analyzed depending on the fulfillment of statistical assumptions and subjected to ANOVA variance analysis followed by a post hoc Tukey test. Nonparametric data were analyzed using Kruskal-Wallis statistics and Dunn’s multiple comparison test. These analysis were performed using Graphpad Prism software version 5.03 (GraphPad software) establishing a p <0.05 value below which the variable values in each treatment were considered significantly different.
RESULTS
Parameters of innate immunity. On the eighth day of the experiment, hens supplemented with 0.1% presented better IF than other groups (Figure 1). By day 30, supplementation with SH at 0.1 and 0.2% resulted in a statistically higher IF (p <0.05) than the non-supplemented groups. At day 30, the IF of the supplemented SH groups decreased (36.8 and 36.9) but compared to non-supplemented groups SH (3.9 and 24.5) which showed a statistically higher IF (p <0.05). In treatment 1 it was visibly noticed that the IF decreased over time, and the eighth day of supplementation showed the highest IF (71.75) while on days 30 and 60 it decreased to 36.8 and 36.1 respectively (Table 1).
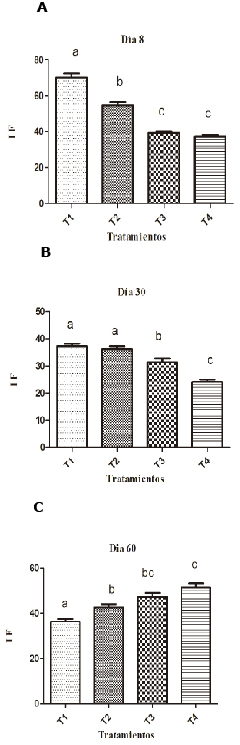
Figure 1. Effect of SH supplements (%) on the phagocytic index (IF) in different treatments (T1, T2, T3 and T4). T1: 0.1%, T2: 0.2%, T3: 0.25 mg/kg of levamisole, T4: without supplement, day 8 (A), 30 (B) and 60 (C). Different letters indicate statistical differences (p<0.05).
Table 1. Phagocytic index, respiratory burst, serum bacteria activity bacterial agglutination in hens with SH
supplements.
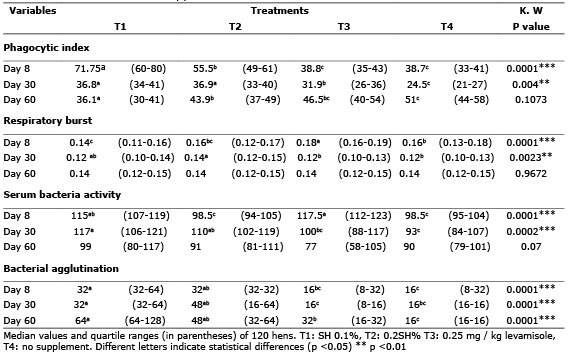
Birds supplemented with 0.1% SH have lower respiratory burst values (p <0.05) than the group supplemented with Levamisole on the eighth day and the negative control group on day 30 of the experiment (Figure 2). The groups supplemented with 0.2% SH had higher respiratory burst values than the unsupplemented groups and on day 60 there were no significant differences (p<0.05) (Table 1).
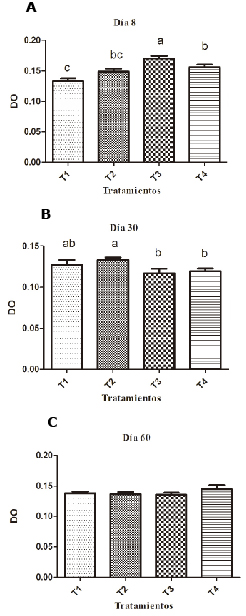
Figure 2. Effect of SH supplements on respiratory burst using the Kruskal Wallis test in different treatments (T1, T2, T3 y T4). T1: 0.1%, T2: 0.2%, T3: 0.25 mg/kg of levamisole, T4: without supplement, day 8 (A), 30 (B) and 60 (C). Different letters indicate statistical differences (p<0.05).
Supplementation with 0.1% SH resulted in a significantly greater ABS than the group supplemented with 0.2% and the non-supplemented group on the eighth day (p <0.05). By day 30, SH supplementation had a significantly greater ABS than the unsupplemented groups (p<0.05), as shown in figure 3.
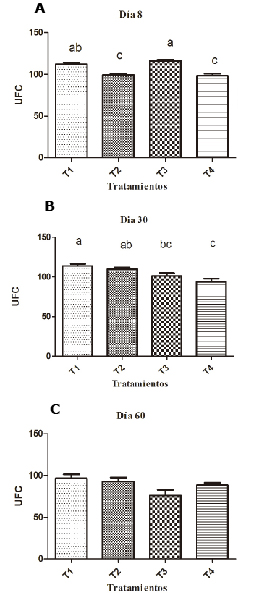
Figure 3. Effect of SH supplements on serum bacteria activity in different treatments (T1, T2, T3 y T4). T1: 0.1%, T2: 0.2%, T3: 0.25 mg/kg of levamisole, T4: without supplement, day 8 (A), 30 (B) and 60 (C). Different letters indicate statistical differences (p<0.05).
Serum bactericidal activity increased on day 30 (p<0.05) and was at the same level on day 60, in which no differences between groups were observed (p>0.05); (Figure 3).
As shown in figure 4, on day 8 bacterial agglutination was higher in the groups supplemented with 0.1% SH than in the other groups (p<0.05).
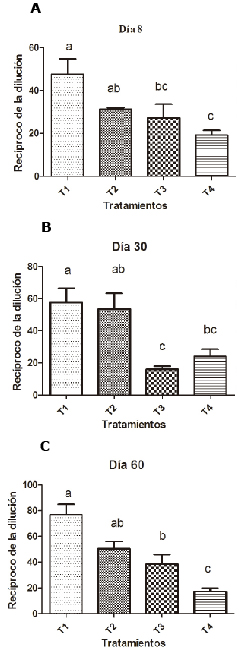
Figure 4. Effect of SH supplements on bacterial agglutination in different treatments (T1, T2, T3 y T4). T1: 0.1%, T2: 0.2%, T3: 0.25 mg/kg of levamisole, T4: without supplement, day 8 (A), 30 (B) and 60 (C). Different letters indicate statistical differences (p<0.05).
On days 30 and 60 it was seen that supplementation with humic substances significantly improves bacterial agglutination activity (p<0.05)
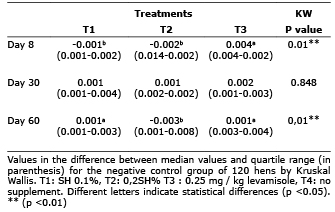
On day 30 of the experiment no significant difference in lysozyme activity was found between treatments when the DO difference relative to the negative control (p> 0.05) was analyzed. On day 60, the group of hens supplemented with 0.1% SH showed a greater difference in the DO regarding the negative control in comparison with the supplemented group with 0.2% SH (p<0.05) (Table 2).
Table 2. Difference in lysozyme activity compared to negative control hens supplemented with SH.
DISCUSSION
Heterophils are phagocytic microbicidal cells that primarily depend on non-oxidative mechanisms for antimicrobial activity, and since they do not contain catalase or myeloperoxidase, this means the production of hydrogen peroxide is much more limited than in mammalian neutrophils (20). Additionally, it is known that macrophages are capable of trapping foreign antigens and initiating an adaptive immune response phase (21). In this study it was found that the SH improve IF on day 8 and 30 of the post-molting phase, but at day 60 is even lower than the unsupplemented groups, so it is understood that the increase in phagocytic activity generated by SH in the early post-molt stage has limited stimulatory functions and for unknown reasons the phagocytes not soon recover their natural activity quickly, and therefore day 60 is significantly different from the control group.
To the authors’ knowledge there are no reports in literature on the effect SH has on these immune parameters. However, similar results were found using other immunostimulatory molecules. Engstad et al (22) demonstrated that human leukocytes increase their immunological activity with β-glucans; however, Chuammitri et al (23) demonstrated that the phagocytic index and bactericidal activity of heterophiles did not show significant differences between those treated with β-glucans and ascorbic acid and those that were untreated, while murine macrophage phagocytosis is increased by Panax ginseng and other immunomodulators (24).
Furthermore, reports show that phagocytic activity is influenced by genetics. Swaggerty et al (25) compared the phagocytic and bactericidal activity of chicken heterophiles in fast and slow feathering. The authors demonstrated that fast-feathering chickens showed a higher phagocytic index and bactericidal activity. Therefore it is suggested study the difference SH has on the immune response according to the different genetic lines existing in broiler and egg production, respectively.
Results found in this work on bacterial agglutination infer that supplementation with SH at 0.1 and 0.2% improves the effects of bacterial agglutination serum in laying hens (p <0.05). Although no published data were found on this species to compare, these results relate to what was presented by Behera et al (18), who conducted a parenteral immunization with glycolic acid on Rohita labeo and reported that the treated group showed a significantly higher difference in bacterial agglutination test on the first day of immunization without obtaining significant differences at days 21 and 42, data that differ from the results presented in this work since SH supplementation significantly improved bacterial agglutination in all sampling (8, 30 60).
Lysozyme activity showed significant improvement on day 60 with a supplementation of 0.1% SH. According to Li et al (26), the egg is nature’s richest source of lysozyme. Hence it is relevant to consider whether the increase in thickness of egg albumin is related to an increase in the proportion of Lysozyme, which is in turn transferred at the egg level to breeding hens.
The blood serum of chickens supplemented with SH had a higher bacterial agglutination during the entire experiment, even surpassing the positive control group; however, publications on this subject that consider previous results have not been found.
Respiratory burst tests showed that at day 30 of the post-molt, hens supplemented with SH had higher oxidative activity. These data agree with Ibuki et al (27), who found that HD11 chicken macrophages treated in vitro with mannobiose increased the production of hydrogen peroxide in comparison to untreated macrophages. Similarly, Babu et al (28) challenged HD11 macrophages with Salmonella enteritidis and found that superoxide anion production was higher than in murine macrophages.
Although forced molting is practiced in production systems to increase the productive life of chickens and improve production and egg quality parameters, there is evidence that in the post-molt stage the relationship between pathogens and host is altered. Therefore, eggs produced in the early post-molt period present a higher risk of contamination for humans since the percentage of contaminated eggs is increased (29). Results reported in this study show that supplementing laying hens in the post-molt phase generated significant increases in IF, lysozyme activity and serum bactericidal activity during the early post-molt stage (Day 8 and 30), whereas the same supplementation in the mid post-molt stage (day 60) decreases IF markedly and does not present stimulatory effects on respiratory burst activity or lysozyme, however bacterial agglutination is stimulated at all measured times.
In conclusion, information in this study suggests that supplementation of laying hens during the post-molt phase with SH from the biotransformation of cachaza, leaf and sugar cane vinasse, can act as stimulants on the parameters of innate immunity evaluated in laying hens particularly in the early stage of this phase, considering that it had higher IF (Day 8 and 30), bacterial agglutination increased during the whole experiment (day 8, 30 and 60), even surpassing the control group, increasing bactericidal activity of the serum on day 8 and 30, and lysozyme activity was significantly better on day 60 of supplementation.
Acknowledgments
The present study was carried out thanks to the financial and logistical contribution at the University of Tolima and the Universidad Cooperativa de Colombia. Thanks to Felipe Velasquez Rodriguez and Adriana Claudia Cely of the Universidad Cooperativa de Colombia and to the Veterinary Diagnostic Laboratory at the Universidad de Tolima.
REFERENCES
1. Van den A, London N, Driessen C, Stobberingh E. Antibiotic resistance of faecal Escherichia coli in poultry, poultry farmers and slaughterers. J Antimicrob Chemoter 2001; 47(6):763-771.
2. Pomorska-Mol L, Pejsak Z. Effects of antibiotics on acquired immunity in vivo current state of knowledge. Pol J Vet Sci 2012; 15(3):583-588.
3. Akhtar M, Tariq A, Awais M, Iqbal Z, Muhammad F, Shahid M, Hiszczynska-Sawicka E. Studies on wheat bran arabinoxylan for its immunoestimulatory and protective effects avian coccidiosis. Carbohydr Polym 2012; 90(1):333-339.
4. Foster N, Berndt A, Lalmanach A, Methner U, Pascquali P, Rychlich I, et al Emergency and therapeutic vaccination - is stimulating innate immunity an option?. Res Vet Sci 2012; 93(1):7-12.
5. Wondmeneh E, Getachew T., Dessie T. Effect of Effective microorganisms (EM®) on the growth parameters of fayoumi and Horro chicken. Int J Poult Sci 2011; 10(3):185-188.
6. Vlcová Z. Chemical and physical transformations of humic acids. [Tesis Doctoral]. BRNO University id technology, Faculty of chemistry, Institute of physical and applied chemistry: Brno, República Checa; 2009.
7. Ergin O, Ocak N, Turan A, Erener G, Altop A, Cankaya S. Performance, carcass, gastrointestinal tract and meat quality traits and selected blood parameters of broilers fed diets supplemented with humic substances. J Sci Food Agric 2011; 92(1):59-65.
8. Eren M, Gezen S, Deniz G, Orhan F. Effects of liquid humate supplemented to drinking water on the performance and eggshell quality of hens in different laying periods. Revue Med Vet 2008; 159(2):91-95.
9. Sanmiguel R, Rondon I. Suplementación con sustancias húmicas en gallinas ponedoras durante la fase posmuda. Rev CES Med Zootec. 2014; 9(2): 169-178.
10. El-Deek A & Al-harthi M. Post molt performance parameters of broiler breeder hens associated with molt induced by feed restriction, high dietary zinc and fasting. Int J Poult Sci 2004; 3(7):456-462.
11. Hassanien H. Effect of force molting programs on egg production and quality of laying hens. Asian J Poult Sci 2011; 5(1):13-20.
12. Tega E, Ibrahim T. Effect of induced molting on fertility and hatchability chickens. Continental J Anim Vet Res 2010; 2(1):31-34.
13. Moreno-Rueda G. Experimental test of a trade of between moult and immune response in house sparrows Passer domesticus. J Evol Biol 2010; 23(10):2229-2237.
14. Akram M, Zia-ur-rahman C, Kim S. Effect on the induced molting on the relative weights and hormone levels of thyroid, ovary and adrenal glands in spent laying hens. Korean J Poult Sci 2002; 29(4):243-247.
15. Millet S, Bennett J, Lee K, Hau M, Klasing K. Quantifying and comparing constitutive across avian species. Dev Comp Immunol 2007; 31(2):188-201.
16. Wells L, Lowry V, Deloach J, Kogut M. Age dependent phagocytosis and bactericidal activities of the chicken heterophil. Dev Comp Immunol 1998; 22(1):103-109.
17. Mohanty B, Sahoo P. Immune response and expression profiles of some imune related genes in Indian major carp, Labeo rohita to Edwardsiella tarda infection. Fish Shellfish Immunol 2010; 28(4):613-621.
18. Behera T, Nanda T, Mohanty C, Mohapatra D, Swain P, Das B, et al. Parenteral immunization of fish, Labeo rohita whit poly D L- lactide Co- glycolic acid (PLGA) encapsulated antigen microparticles promotes innate and adaptive immune responses. Fish Shellfish Immunol 2010; 28(2):320-325.
19. Low K, Sin P. In vivo and in vitro effects of mercuric chloride and sodium selenite on some non-specific immune responses of blue gourami, Thricogaster trichopterus (Pallus). Fish Shellfish Immunol 1996; 6(5):351-362.
20. Harmon B. Avian heterophils in inflammation and disease resistance. Poult Sci 1998; 77: 972-977.
21. Lim TS, Na K, Choi EM, Chung JY, Hwang JK, Immunomodulating activities of polysaccharides isolated from Panax ginseng. J Med Food 2004; 7(1):1–6.
22. Engstad C, Engstad R, Olsen J, Osterud B. The effect of soluble -1,3-glucan and lipopolysaccharide on cytokine production and coagulation activation in whole blood. Int J Immunopharmacol 2002; 2(11):1585–1597.
23. Huammitri P, Redmond S, Kimura K, Andreasen C, Lamont S, Palic D. Heterophil functional responses to dietary immunomodulators vary in genetically distinct chicken lines. Vet immunol immunopathol 2011; 142(3):219-227.
24. Jiao l, Wank D, Zhang X, Li B, Zhao H, Liu S. Characterization and immunostimulating effects on murine peritoneal of oligosaccharide isolated from Panax ginseng C.A Meyer. J Ethnopharmacol 2012; 144(3):490-496.
25. Swaggerty C, Pevzner I, Ferro P, Crippen T, Kogut M. Association between in vitro heterophil function and the feathering gene in commercial broiler chickens. Avian Pathol 2003; 32(5):433-438.
26. Li B, Y Huang, Paskewitz S. Hen egg white lysozyme as an inhibitor of mushroom tyrosinase. FEBS Letter 2006; 580(7):1877-1882.
27. Ibuki M, Kovacs-Nolan J, Fukui K, Kanatani H, Mine Y. Β 1-4 mannobiose enhances Salmonella Killing activity and activate innate immune responses in chicken macrophages. Vet Immunol Immunopathol 2011; 139(2-4):289-295.
28. Babu U, Gaines D, Lillehoj H, Rayburn R. Differential reactive oxygen and nitrogen production and clearance of Salmonella serovars by chicken and mouse macrophages. Dev comp immunol 2006; 30(10);942-953.
29. Woodward C, Kwon Y, Kubena N, Byrd J, Moore R, Nisbet D, Ricke S. Reduction of Salmonella enterica serovar enteritidis colonization and invasion by an alfalfa diet during molt in leghorn hens. Poult Sci 2005; 84(2):185-193.





